Work in the ScleroLab is patient-oriented, bench-to-bedside, and encompasses several approaches, ranging from genetics and functional genomics to vascular biology, cellular immunology, metabolic studies, molecular and live imaging, and matrix biology. We deploy these approaches to discover, validate, and develop novel targets for diagnosis, precision medicine, and targeted human therapies. Our current research projects are listed below.
The overarching theme of our future work is to understand how gene-environment interactions trigger and sustain fibrosis, and to mine this knowledge for drug discovery. We will pursue four linked areas:
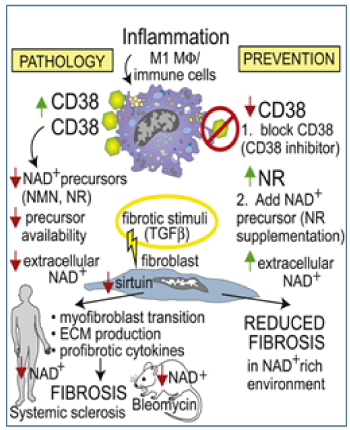
- (dys)regulation of NAD+ metabolism linking inflammation, vascular injury and fibrosis in scleroderma
- genetic polymorphisms such as A20 (TNFAIP3) shaping immunity and matrix remodeling in health and disease
- diet and gut microbiome-derived metabolites, such as trimethylamine oxide (TMAO), driving systemic immune, vascular and fibrotic responses
- a novel pathogenic acquired primary ciliopathy in scleroderma
Our work in each of these areas is cross-disciplinary, patient-oriented, and therapy-focused. We will make extensive use of human disease samples and clinical information collected from our rheumatology clinics at Michigan Medicine. We will identify and functionally characterize endophenotype-associated common and rare genetic variants; study gene expression in biopsies using bulk and single-cell RNA-seq and ATAC-seq, spatial transcriptomics and metabolomics; and optimize disease models including cellular assays, organoids and engineered mice. We will collaborate with investigators from several departments using cross-cutting approaches of computational biology, cell biology, immunology, and bioengineering.
Scleroderma, along with lupus and other chronic rheumatic diseases, is characterized by synchronous injury and damage in the immune, vascular and stromal compartments throughout the body. Understanding and treating these conditions, therefore, requires integrated “organ-agnostic” cross-cutting scientific collaborations.
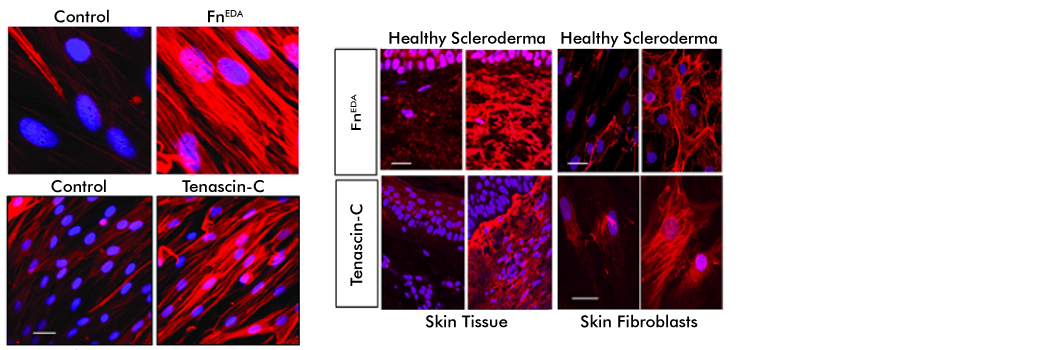
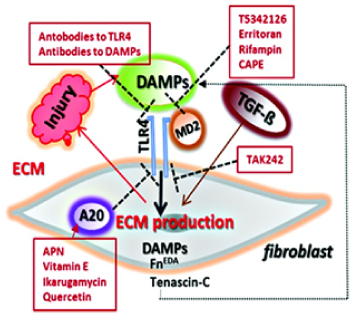
DAMPs: Damage-Associated Molecular Patterns Driving Fibrosis Progression in Scleroderma
(Sponsor: NIH; R01 AR074997)