Identifying and developing novel outcomes and endpoints is critical to best evaluate patient health and care. Relevant, achievable endpoints and outcomes include both clinical endpoints (e.g. kidney function, proteinuria) and clinical outcomes assessments (e.g. patient-reported outcomes). As a co-founder of the Nephrotic Syndrome PRO Consortium, the UM Pediatric Nephrology Research Program strives for advancement in acceptable outcomes and endpoints for kidney disease patients.

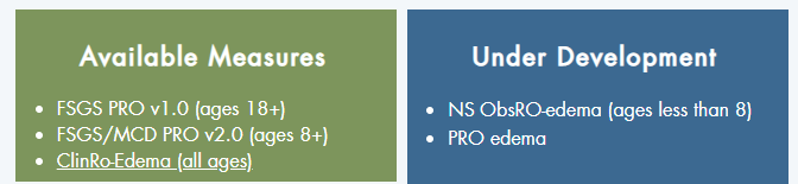
Prepare-NS is a program to develop and validate publicly available clinical outcome assessments (COAs) fit for drug development for swelling from nephrotic syndrome associated kidney diseases. Learn more at https://www.prepare-ns.org
The Nephrotic Syndrome Patient Reported Outcomes Consortium is a collaboration between academia, patient foundation partners, and industry to develop measures that collect information directly from patients about how they feel and function. These measures can be used in clinical and research settings. Learn more at www.kidneyresearchnetwork.org/patient-reported-outcomes