Lorraine Buis, Ph.D., assistant professor and health technologist, is leading a new randomized controlled trial that aims to support African American patients with uncontrolled hypertension in their self-management of the disease with a low-cost, multi-pronged mobile health (mHealth) application called MI-BP. The trial's research protocol was recently published in the Journal of Medical Internet Research (JMIR) Protocols.
From the protocol, Buis notes that "African Americans shoulder significant disparities related to hypertension (HTN), which is a serious public health problem in the city of Detroit, Michigan, where more than 80% of the population is African American. Connectivity through smartphones, use of home blood pressure (BP) monitoring, and newly developed mobile health (mHealth) interventions can facilitate behavioral changes and may improve long-term self-care for chronic conditions."
The protocol authors add that "implementation of a combined approach utilizing these methods has not been tested among African American patients with uncontrolled HTN. Since African Americans are more likely than other racial or ethnic subgroups to utilize the emergency department (ED) for ambulatory care, this presents an opportunity to intervene on a population that is otherwise difficult to reach."
SEE ALSO: Buis pens Detroit Free Press op ed on Medicare Advantage coverage of low cost health technology
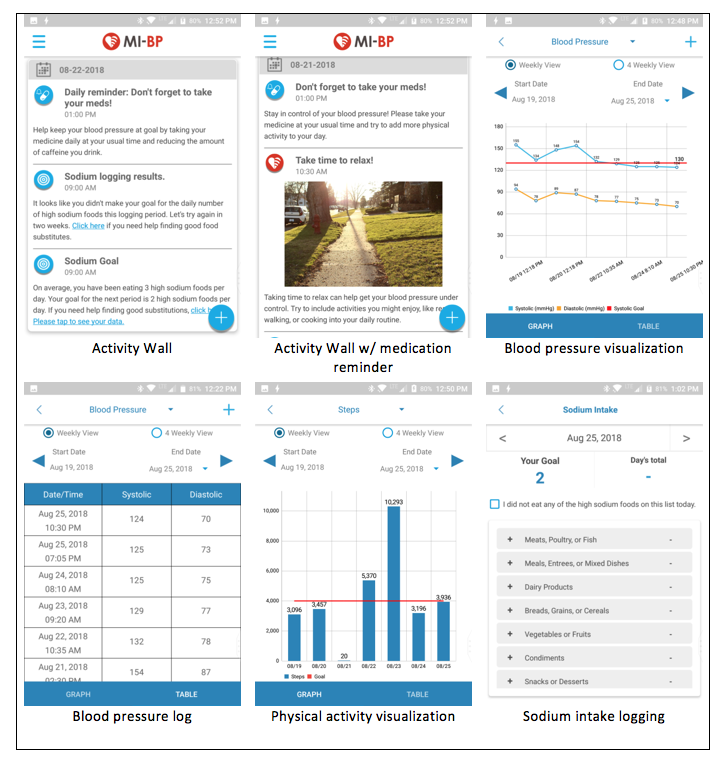
Buis and her research team worked with Vibrent Health to develop MI-BP, a multifactor mobile health application that allows users to sync and track blood pressure readings with compatible Bluetooth blood pressure cuff, to log physical activity and set gradual step goals, and to track dietary sodium intake. Users interact with the application through the interface and through mobile push notifications. According to the authors, the app "aims to reduce health disparities related to HTN in the community by employing a user-centered intervention focused on self-BP monitoring, physical activity, reduced sodium intake, and medication adherence." Below is a composite of screenshots from the MI-BP app.
- Screenshots of MI-BP app, a mobile health intervention developed by Lorraine Buis et al. Image accessed from the study's protocol, published in JMIR Research Protocol
The study is currently recruiting African American participants, ages 25 to 70, with uncontrolled hypertension from two emergency department sites in Detroit, Michigan (NCT02360293). It's the first of its kind to be conducted in an ED setting and was designed to be accessible to a population that might be otherwise difficult to reach. The major aims of the trial are to:
- Determine the effect of MI-BP on blood pressure (BP) for 1 year, using BP control and mean systolic blood pressure (SBP) as co-primary outcomes) compared with usual care controls, in a 1-year randomized controlled trial (RCT)
- Determine the effect of MI-BP on secondary outcomes: physical activity, sodium intake, and medication adherence compared with usual care
- Evaluate the cost-effectiveness of MI-BP compared with usual care
The MI-BP trial is a large-scale extension of BP-MED, a pilot text messaging intervention developed by Buis to assist in the self management of uncontrolled hypertension.
SEE ALSO: Text Message Alerts Help Patients with Hypertension Remember to Take Meds
Article citation: Buis LR, Dawood K, Kadri R, Dawood R, Richardson CR, Djuric Z, Sen A, Plegue M, Hutton D, Brody A, McNaughton CD, Brook RD, Levy P. Improving Blood Pressure Among African Americans With Hypertension Using a Mobile Health Approach (the MI-BP App): Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2019;8(1):e12601. DOI: 10.2196/12601
Browse the latest clinical informatics and technology and preventive medicine research from the department of family medicine.