
Kevin Chen, M.D., a clinical assistant professor in both neurology and neurosurgery, has been named the new Handleman Emerging Scholar at Michigan Medicine’s NeuroNetwork for Emerging Therapies.
“We cannot thank Charlene enough for her continued support of the work of our laboratory and her belief in the importance of encouraging the careers of promising scientists like Dr. Chen,” says Dr. Eva Feldman, director of the NeuroNetwork. “His work is on the cutting edge of research, with great potential to further our understanding of neurologic disease and to lead to novel therapeutic approaches.”
The incredible generosity of the award’s namesake, longtime NeuroNetwork supporter Charlene Handleman, will allow Dr. Chen to advance his groundbreaking work utilizing the unique properties of stem cells to understand what causes neurodegenerative diseases, like ALS and Alzheimer’s disease, and advancing the use of the stem cells themselves as a potential treatment agent for such devastating diseases.

This is the kind of work Charlene Handleman envisioned when she began to fund an emerging scholar.
“I am very interested in brain health. Understanding why someone develops a brain disease and what can be done to offer treatment and hope is important to me. I am so pleased Dr. Chen is the newly appointed Handleman Emerging Scholar, and I look forward to following his research.”
We spoke to Dr. Chen about this support, his career, and what it will mean to his research ambitions.
The Path to Neuroscience and ALS:
Dr. Chen recalls that during his high school years, he asked himself big questions – what it means to be a person and what it is that makes us human. “Those thoughts led to an interest in the neurosciences,” explains Dr. Chen, “because the brain is the seed of the soul.”
“It was the first time I was not just reading out of a textbook but asking questions and designing experiments to figure out the next answer,” says Chen. “That was also where I was introduced to ALS.”He left home to study anthropology and neuroscience at Johns Hopkins University. His freshman-year Introduction to Neuroscience professor, Dr. Stewart Hendry, offered him the opportunity to be involved in research projects. He was paired with Dr. Lee Martin, a neuropathologist who was studying ALS.
And, Neurosurgery?
Dr. Chen next enrolled at the Duke University School of Medicine, not knowing for certain in which field he wanted to specialize, describing himself as “one of those people trying to decide between neurology and neurosurgery.” He chose neurosurgery because, at the time, it felt more immediate and more tangible. Surgeons could see a tumor on a scan. Surgeons could see the results of their work once the patient woke up. In contrast, neurology, while highly intellectual, could be more abstract or ephemeral, with diagnoses that were less defined and, at times, limited in treatment options.
“When I was in medical school there weren’t treatments for many diseases,” says Dr. Chen. “For example, I think for multiple sclerosis there was one drug; for Alzheimer’s, maybe one or two. In the case of many conditions, it was like, ‘Oh, we figured out what you have, but we can’t do anything.’ That’s what steered me towards neurosurgery.” In neurosurgery, he could work with his hands, but still, incorporate this fascination with the nervous system.
He goes on to explain: “For me, it’s always been about the neuroscience, the brain function, and things like your speech, your memory, your movements. These are the things we think about when we plan surgery on the brain or spinal cord.”
The Road to ALS Research:
“Even though thousands of researchers have worked on these diseases for decades, the introductory paragraphs of our papers begin the same, ‘The causes of ALS are not understood, the causes of Alzheimer’s are not understood.’ There is so much more we need to figure out and understand.”Dr. Chen always had an interest in ALS and neurodegenerative disease since his undergraduate days. He feels neurodegenerative disease remains largely a mystery.
In most areas of medicine, Dr. Chen believes there is some “other” thing you can blame: a tumor, an aneurysm or diabetes. However, with ALS or Alzheimer's, it's our own brains or bodies that fail us. Fighting those diseases, to Dr. Chen, felt more meaningful and motivated him to look for a way to return to the study of ALS.
He found the opportunity when he became a neurosurgery resident at Michigan Medicine. Dr. Eva Feldman was beginning the first clinical trial involving the transplantation of stem cells into the spinal cords of ALS patients and was looking for residents to join the project. Dr. Chen jumped at the chance.
Why Stem Cells Are an Intriguing Target:
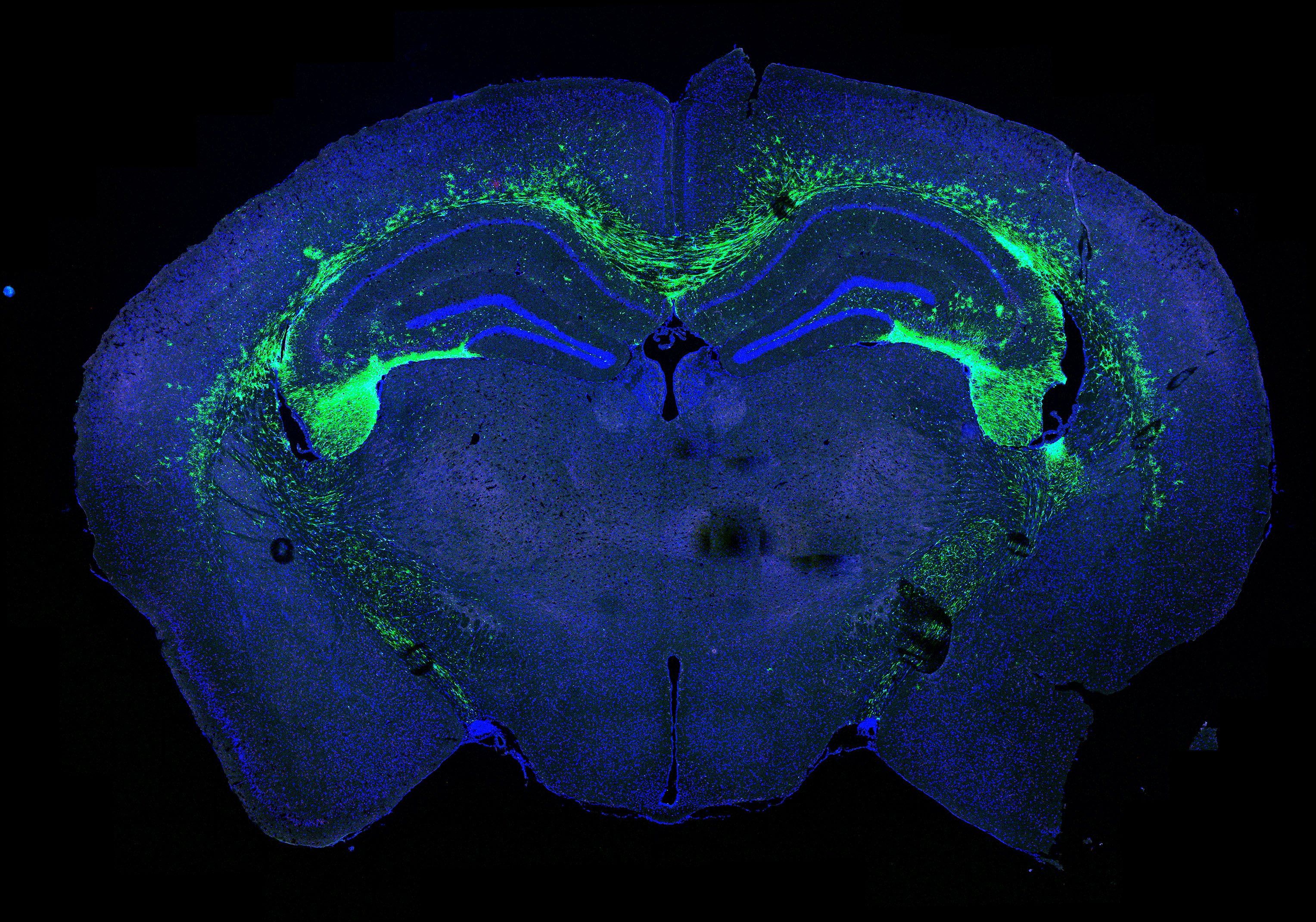
Stem cells allow researchers to explore the causes of diseases because they develop into a host of different cell types, such as neurons, and can then be manipulated in a way that isn’t possible with drugs and other therapies. Dr. Chen likens them to “micro-machines,” whose biology can be harnessed to produce what is needed—Insulin-like Growth Factor 1, antibodies, etc.—obviating the use of IV’s, pumps and other external measures, the extended use of which can cause a host of problems.
Charlene Handleman’s Gift Will Directly Support:
Dr. Chen’s “midterm” goals are to employ the latest technologies to home in on interneurons to understand how they might be disrupted in patients with ALS and Alzheimer’s disease. One of the hurdles is learning to tease out the specific subtypes of interneurons researchers are interested in studying from all the others in the brain. To accomplish this, Dr. Chen is seeking to employ spatial transcriptomics, a very new technology, that can detect gene expression in particular regions of the brain and spinal cord.
In the immediate future, Dr. Chen plans to continue the research begun by the inaugural Handleman Emerging Scholar, Dr. Lisa McGinley, examining animal models of Alzheimer’s disease models that have undergone stem cell transplantations to understand the mechanisms that allow these stem cells to improve function. In particular, he will explore how to modify stem cells to increase their effectiveness as a therapy target in Alzheimer’s disease. In ALS, he will study inhibitory neurons and synapses to better understand the role they might play in reversing disease progression.
In addition, Dr. Chen and his colleagues will also attempt a novel approach using Induced Pluripotent Stem Cells (iPS) derived from ALS and Alzheimer's patients. At the moment, there isn’t a well-established method of turning these iPS stem cells into interneurons. Once that can be realized, the scientists will be able to model interneurons in a dish, where they can be manipulated more easily.
His Personal Medical Moonshot:
“We are creating a process by which we can take a patient’s cells, like skin cells, reprogram them to become induced pluripotent stem cells that will differentiate into interneurons, while along the way maybe correcting some of the deficits we see contributing to ALS or Alzheimer’s disease,” explains Dr. Chen.
“These ‘corrected’ interneurons could then be transplanted back into the patient, where they hopefully would regenerate or restore these inhibitory neurons or synapses to slow disease progression.”
Is this result possible in our lifetime?
“The exciting thing is that this builds on work we can already do,” says Dr. Chen. “We have the creation of the iPS stem cell lines down pat. We are one of the only labs that can conduct intracerebral and intraspinal transplantations in humans. So, it’s really only the middle part we need to figure out to bridge the process -- finding out what went wrong with interneurons and then fixing them so they can be retransplanted.”
“I believe in Dr. Chen, and treatments that decade ago seemed impossible, are now within our reach. I want a front row seat in his medical moonshot rocket! I believe together we can make a real difference in the lives of patients with Alzheimer’s disease and ALS.”

The Importance of Philanthropy:
“When I heard the good news, I was equal parts shocked, honored and surprised,” reports Dr. Chen. “These named honors usually go to senior doctors and researchers, so as a junior faculty, it was a great feeling. I’m so grateful for Charlene’s support because it enables me to take risks and explore where the research takes me and, hopefully, make discoveries that change lives.”
