Abstract
X-chromosome inactivation is a paradigm of epigenetic transcriptional regulation. Female human embryonic stem cells (hESCs) often undergo erosion of X-inactivation upon prolonged culture. Here, we investigate the sources of X-inactivation instability by deriving new primed pluripotent hESC lines. We find that culture media composition dramatically influenced the expression of XIST lncRNA, a key regulator of X-inactivation. hESCs cultured in a defined xenofree medium stably maintained XIST RNA expression and coating, whereas hESCs cultured in the widely used mTeSR1 medium lost XIST RNA expression. We pinpointed lithium chloride in mTeSR1 as a cause of XIST RNA loss. The addition of lithium chloride or inhibitors of GSK-3 proteins that are targeted by lithium to the defined hESC culture medium impeded XIST RNA expression. GSK-3 inhibition in differentiating female mouse embryonic stem cells and epiblast stem cells also resulted in a loss of XIST RNA expression. Together, these data may reconcile observed variations in X-inactivation in hESCs and inform the faithful culture of pluripotent stem cells.
Introduction
Human pluripotent stem cells (hPSCs) offer the possibility to model early human development in vitro and are substrates for regenerative medicine1,2,3. The promise of hPSCs relies on their ability to faithfully maintain their epigenetic and transcriptional profiles in culture.
X-chromosome inactivation is a paradigm of epigenetic transcriptional regulation that equalizes X-linked gene expression between female and male mammals4,5,6. Once inactivated, with a few key exceptions, replicated copies of the silenced X chromosome remain stably inactive in descendant cells7. X-inactivation is an experimentally tractable system to interrogate epigenetic transcriptional regulation because two equivalent X chromosomes become transcriptionally divergent and these divergent transcriptional states are subsequently stably transmitted across mitotic cell division.
X-inactivation has been studied extensively in mouse embryos and mouse embryonic stem cells (mESCs). In the female preimplantation mouse embryo, all cells undergo imprinted inactivation of the paternal X chromosome8,9,10. At the peri-implantation stage, the inactivated paternal X chromosome is reactivated in the pluripotent epiblast progenitor cells11,12. Conventionally cultured mESCs capture this transient population of pluripotent cells and harbor two active X chromosomes13. Upon differentiation, pluripotent mouse embryonic epiblast cells as well as mESCs inactivate either the maternal or the paternal X chromosome in individual cells in a process termed random X-inactivation13,14,15,16,17,18.
In contrast to the mouse, human female preimplantation embryos do not undergo imprinted inactivation of the paternal X chromosome19,20,21,22. Instead, both X chromosomes in female preimplantation human embryos appear to initiate some degree of silencing19,20,21,22. The X chromosome-encoded long noncoding XIST RNA, whose expression is a hallmark of the inactive X chromosome and is required for stable X-inactivation in mice10,23, is expressed from and coats in cis both X chromosomes in most cells of female human blastocyst-stage embryos19,20,21. XIST RNA coating in turn recruits a diverse array of proteins to the X chromosome that silence gene expression24,25,26,27,28. Despite XIST RNA coating of the X chromosomes, however, X-linked genes are not fully silenced in preimplantation human embryos19,20,21.
Unlike female mESCs, which harbor two activeXs13, female human ESC (hESC) lines display variable patterns of X-inactivation22,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49. This variability appears to reflect both differences in X-inactivation patterns in early mouse vs. human female embryos and potentially how hESCs are derived and cultured.
The pattern of X-inactivation in early preimplantation human embryos is partially recapitulated in vitro through the derivation of ‘naïve’ pluripotent female hESCs22,46,47,48,49,50. In these naïve hESC lines, a proportion of cells display XIST RNA coating of both X chromosomes but do not appear to transcriptionally inactivate the XIST RNA-coated Xs, similar to cells in early female human embryos. Most female hESCs cultured in naïve conditions, however, harbor one XIST RNA-coated X chromosome that is transcriptionally active22,46,48,49,50. The heterogeneity of XIST RNA expression in naïve female hESCs appears to be due to the coexistence in culture of at least two populations of pluripotent cells47. Blocking autocrine basic fiborblast growth factor (bFGF) signaling reduces this heterogeneity and is reported to yield nearly all hESCs with two XIST RNA-coated X chromosomes47, recapitulating the pattern observed in epiblast cells of preimplantation female human embryos20,21. Differentiation of these naïve hESCs into the ‘primed’ pluripotent hESCs results in a majority of the cells undergoing random X-inactivation and exhibiting XIST RNA coating of a single X chromosome that is transcriptionally inactive47.
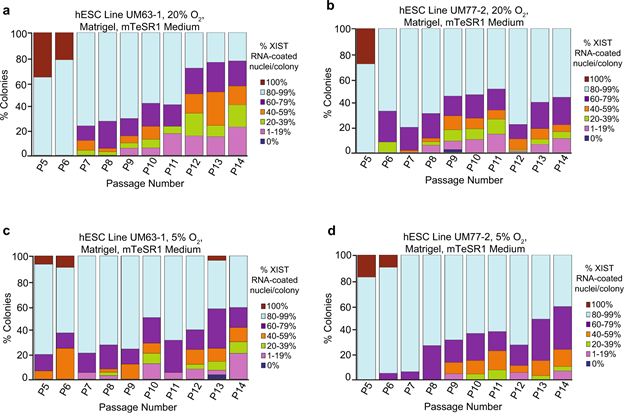
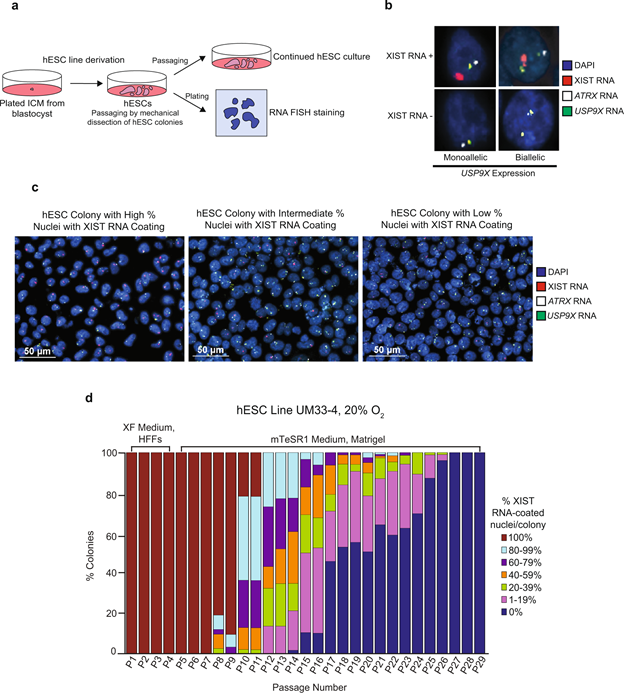
Compared to naïve hESCs, primed pluripotent hESCs capture a later stage of embryonic development and may be analogous to mouse epiblast stem cells (mEpiSCs)3,51,52,53,54,55,56. Female mEpiSCs contain one inactivated, XIST RNA-coated X chromosome14. Primed female hESCs, by contrast, exhibit at least three patterns of X-inactivation. Primed female hESCs can harbor no inactive X chromosome, one inactive-X, or a leaky inactive-X29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,45. Upon prolonged culture, many primed female hESC lines lose XIST RNA coating, which is accompanied by the expression of a subset of previously silenced genes from the inactive X chromosome29,31,35,36,38,39,43,45,57,58. This loss of XIST RNA coating and re-expression of silenced X-linked genes has been termed X-inactivation erosion31,34,35,38,42,43,58. Loss of XIST RNA coating and the leaky expression of inactive X-linked genes also characterize cultured human induced pluripotent stem cells (hiPSCs) and are thought to be irreversible35,36,42,45,57,59. Increased X-linked gene expression due to X-inactivation erosion or failure can be deleterious to development and differentiation23,45,60. The instability of the epigenetically inactivated X chromosome lends caution to the use of pluripotent female human cells in disease modeling and regenerative medicine.
The underlying reasons for XIST RNA loss and X-inactivation instability in cultured hESCs are unclear. To gain insights into changes in X-inactivation in hESCs, we derived new hESC lines and analyzed expression of XIST RNA and other X-linked genes in these hESCs under diverse culture conditions and across many passages. Our results implicate the presence of lithium chloride or other GSK-3 inhibitors in the culture media as a cause of XIST RNA expression loss in female hESCs.
Preventing erosion of X-chromosome inactivation in human embryonic stem cells - Full Text
Marissa Cloutier1,†, Surinder Kumar1,†#, Emily Buttigieg1,†, Laura Keller2-5, Brandon Lee1, Aaron Williams1, Sandra Mojica-Perez2-5, Indri Erliandri2-5, Andre Monteiro Da Rocha2-6, Kenneth Cadigan7, Gary D Smith2-5, and Sundeep Kalantry1* 1Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 2Department of Molecular & Integrative Physiology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 3Department of Obstetrics & Gynecology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 4Department of Urology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 5Department of Physiology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 6Department of Internal Medicine & Cardiology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA 7Department of Molecular, Cellular, and Developmental Biology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA #Current affiliation: Department of Pathology, University of Michigan Medical School, Ann Arbor, MI, 48109, USA †Equal contribution *Correspondence: [email protected]
Cloutier, M., Kumar, S., Buttigieg, E. et al. Preventing erosion of X-chromosome inactivation in human embryonic stem cells. Nat Commun 13, 2516 (2022). https://doi.org/10.1038/s41467-022-30259-x



